How Is Decane Cracked To Produce Propane
How Is Decane Cracked To Produce Propane' title='How Is Decane Cracked To Produce Propane' /> Fixed Gas Detectors. Crowcon manufacture a complete range of both fixed and portable gas detection equipment for personal and plant protection against gas hazards across the globe. Based on a solid foundation of expertise in understanding of the core physics and chemistry of gas detection, Crowcon works closely with international bodies to ensure products are designed to industry leading equipment certification and performance standards. WHAT IS GAS The name gas comes from the word chaos, which neatly summarises the main feature of the simplest state of matter. A gas is a swarm of particles moving randomly and chaotically, constantly colliding with each other and the walls of any container. The real volume of the particles is minute compared to the total space which they occupy and this is why gases fill any available volume and are readily compressed Confined space. Crowcon manufacture a complete range of both fixed and portable gas detection equipment for personal and plant protection against gas hazards across the globe. GKL110341Jun15E3 CH1FP Jun15CH1FP01 General Certificate of Secondary Education Foundation Tier June 2015 Science A CH1FP Unit Chemistry C1 Chemistry. Butadiene is a simple conjugated diene with the formula C 4 H 6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. KarthikT., Dhanya Radhakrishanan, Chandrabhas Narayana and Saket Asthana, Nature of electric field driven ferroelectric phase transition in leadfree Na12Bi1. Glossary of terms and acronyms used in Petroleum and Lubricant Industry. The average speeds of gas molecules are of the order of 1. This is why gases mix rapidly and why they exert pressure. This constant motion is easily demonstrated by releasing a small amount of odorous gas in a room. Within seconds the gas can be smelt in all parts of the room. These properties apply to substances, which are normally gaseous, and to vapours from evaporated liquids. ALKANES+INTRODUCING+ALKANES.jpg' alt='How Is Decane Cracked To Produce Propane' title='How Is Decane Cracked To Produce Propane' />
Fixed Gas Detectors. Crowcon manufacture a complete range of both fixed and portable gas detection equipment for personal and plant protection against gas hazards across the globe. Based on a solid foundation of expertise in understanding of the core physics and chemistry of gas detection, Crowcon works closely with international bodies to ensure products are designed to industry leading equipment certification and performance standards. WHAT IS GAS The name gas comes from the word chaos, which neatly summarises the main feature of the simplest state of matter. A gas is a swarm of particles moving randomly and chaotically, constantly colliding with each other and the walls of any container. The real volume of the particles is minute compared to the total space which they occupy and this is why gases fill any available volume and are readily compressed Confined space. Crowcon manufacture a complete range of both fixed and portable gas detection equipment for personal and plant protection against gas hazards across the globe. GKL110341Jun15E3 CH1FP Jun15CH1FP01 General Certificate of Secondary Education Foundation Tier June 2015 Science A CH1FP Unit Chemistry C1 Chemistry. Butadiene is a simple conjugated diene with the formula C 4 H 6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. KarthikT., Dhanya Radhakrishanan, Chandrabhas Narayana and Saket Asthana, Nature of electric field driven ferroelectric phase transition in leadfree Na12Bi1. Glossary of terms and acronyms used in Petroleum and Lubricant Industry. The average speeds of gas molecules are of the order of 1. This is why gases mix rapidly and why they exert pressure. This constant motion is easily demonstrated by releasing a small amount of odorous gas in a room. Within seconds the gas can be smelt in all parts of the room. These properties apply to substances, which are normally gaseous, and to vapours from evaporated liquids. ALKANES+INTRODUCING+ALKANES.jpg' alt='How Is Decane Cracked To Produce Propane' title='How Is Decane Cracked To Produce Propane' />
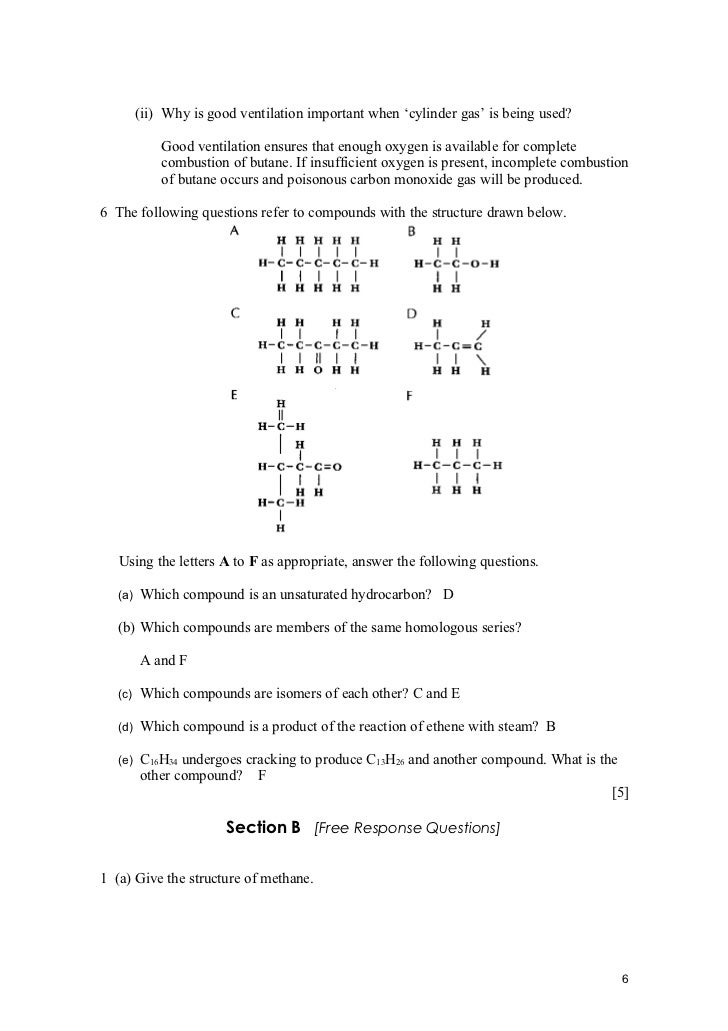 A volume of any gas at the same temperature and pressure contains the same number of molecules irrespective of what the gas is. This means that measuring gas by volume is very convenient. Gas measurements at higher levels are in volume and at lower levels parts per million, ppm volume. Whilst different gases have different densities, they do not totally separate into layers according to their density. Heavy gases tend to sink and light gases tend to rise, but their constant motion means that there is continuous mixing i. This is why it is imperative to locate your Crowcon gas detecting sensors at the right location depending on the gas required to detect. So in a room where there is a natural gas methane leak, the gas will tend to rise because it is lighter than air but the constant motion means that there can be some concentration at floor level. This will happen in perfectly still conditions but if there are any air currents, the mixing will be increased. Air is a mixture of gases, typically Nitrogen 7. Oxygen 2. 0. 9 Standard that is set on all Gas MonitorsWater Vapour 0. Argon 0. 9Carbon Dioxide 0. Other Gases 0. 0. Because its composition is reasonably constant, air is usually considered as a single gas, which simplifies the measurement of toxic and flammable gases for safety and health applications. So for any assistance regarding the Crowcon Gas Detection family, please contact us at Cebeco on 0. Crowcon Gas Detection. FLAMMABLE RISK and COMBUSTIBLE GASESCombustion of Gases and Vapours Most organic chemical compounds will burn. Burning is a simple chemical reaction in which oxygen from the atmosphere combines rapidly with a substance, producing heat. The simplest organic compounds are those known as hydrocarbons and these are the main constituents of crude oilgas. They are composed of carbon and hydrogen, the simplest hydrocarbon being methane, each molecule of which consists of one carbon atom and four hydrogen atoms. It is the first compound in the family known as alkanes. The physical properties of alkanes change with increasing number of carbon atoms in the molecule, those with one to four being gases, those with five to ten being volatile liquids, those with 1. Longer carbon chain hydrocarbons are tars and waxes. The first ten alkanes are CH4 Methane gas C6. H1. 4 Hexane liquidC2. H6 Ethane gas C7. H1. 6 Heptane liquidC3. H8 Propane gas C8. H1. 8 Octane liquidC4. H1. 0 Butane gas C9. H2. 0 Nonane liquidC5. H1. 2 Pentane liquid C1. H2. 2 Decane liquidCrowcon have a gas monitor to suit all applicationsThe above compounds are all known as aliphatics. Alkenes are similar but their molecular structure includes double bonds. Examples are ethylene and propylene. Alkynes contain triple bonds. Example is acetylene Aromatic hydrocarbons such as benzene have a ring molecular structure and burn with a smoky flame. When hydrocarbons burn they react with oxygen from the atmosphere to produce carbon dioxide and water although if the combustion is incomplete because there is insufficient oxygen, carbon monoxide will result as well. More complex organic compounds contain elements such as oxygen, nitrogen, sulphur, chlorine, bromine or fluorine and if these burn, the products of combustion will include other compounds as well. For example substances containing sulphur such as oil or coal will result in sulphur dioxide whilst those containing chlorine such as methyl chloride or polyvinyl chloride PVC will result in hydrogen chloride. In most industrial environments where gas detection is required, there is the risk of explosion or fire because of the presence of flammable gases or vapours, a mixture of compounds is likely to be encountered. In the petrochemical industry, the raw materials are a mixture of chemicals many of which are decomposing naturally or are being altered, by the processes. Saints Row 2 Full Rip Torrent here. For example crude oil is cracked to produce many simpler materials. Explosions. In order for gas to ignite there must be an ignition source typically a spark, or flame or hot surface. For ignition to take place there must be an explosive mixture. This means the concentration of gas or vapour in air must be at a level such that the fuel and oxygen can react chemically. The power of the explosion depends on the fuel and its concentration in the atmosphere. This is why you would use a Crowcon Combustible Gas Monitor to ensure safe levels are kept to protect staff, equipment and the surrounding environment. Not all concentrations of flammable gas or vapour in air will burn or explode. The LOWER EXPLOSIVE LIMITLEL The lowest concentration percentage of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source arc, flame, heat. The term is considered by many safety professionals to be the same as the lower flammable limit LFL. At a concentration in air lower than the LEL, gas mixtures are too lean to burn. Portable gas detectors in Australia have set 5LEL as its first alarm. The UPPER EXPLOSIVE LIMIT UEL Highest concentration percentage of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source arc, flame, heat. Concentrations higher than UFL or UEL are too rich to burn. Flammable liquids generally have a low FLASH POINT. This is the lowest temperature at which vapour is given off at sufficient rate to form an explosive mixture with air. Liquids with flash points below normal ambient temperatures automatically release vapour in sufficient volume to provide an explosive mixture, so leakage of such liquids is potentially as dangerous as a flammable gas leak. VAPOUR DENSITY is a measure of the density of a gas or vapour relative to air. Gases or vapours with a vapour density of less than one are lighter than air and they tend to rise from the point of escape and may therefore be readily dispersed or they may be trapped at a higher level. Gases or vapours with a vapour density of greater than one are heavier than air and tend to sink to lower levels and will spread around forming concentrations between the LEL and UEL.
A volume of any gas at the same temperature and pressure contains the same number of molecules irrespective of what the gas is. This means that measuring gas by volume is very convenient. Gas measurements at higher levels are in volume and at lower levels parts per million, ppm volume. Whilst different gases have different densities, they do not totally separate into layers according to their density. Heavy gases tend to sink and light gases tend to rise, but their constant motion means that there is continuous mixing i. This is why it is imperative to locate your Crowcon gas detecting sensors at the right location depending on the gas required to detect. So in a room where there is a natural gas methane leak, the gas will tend to rise because it is lighter than air but the constant motion means that there can be some concentration at floor level. This will happen in perfectly still conditions but if there are any air currents, the mixing will be increased. Air is a mixture of gases, typically Nitrogen 7. Oxygen 2. 0. 9 Standard that is set on all Gas MonitorsWater Vapour 0. Argon 0. 9Carbon Dioxide 0. Other Gases 0. 0. Because its composition is reasonably constant, air is usually considered as a single gas, which simplifies the measurement of toxic and flammable gases for safety and health applications. So for any assistance regarding the Crowcon Gas Detection family, please contact us at Cebeco on 0. Crowcon Gas Detection. FLAMMABLE RISK and COMBUSTIBLE GASESCombustion of Gases and Vapours Most organic chemical compounds will burn. Burning is a simple chemical reaction in which oxygen from the atmosphere combines rapidly with a substance, producing heat. The simplest organic compounds are those known as hydrocarbons and these are the main constituents of crude oilgas. They are composed of carbon and hydrogen, the simplest hydrocarbon being methane, each molecule of which consists of one carbon atom and four hydrogen atoms. It is the first compound in the family known as alkanes. The physical properties of alkanes change with increasing number of carbon atoms in the molecule, those with one to four being gases, those with five to ten being volatile liquids, those with 1. Longer carbon chain hydrocarbons are tars and waxes. The first ten alkanes are CH4 Methane gas C6. H1. 4 Hexane liquidC2. H6 Ethane gas C7. H1. 6 Heptane liquidC3. H8 Propane gas C8. H1. 8 Octane liquidC4. H1. 0 Butane gas C9. H2. 0 Nonane liquidC5. H1. 2 Pentane liquid C1. H2. 2 Decane liquidCrowcon have a gas monitor to suit all applicationsThe above compounds are all known as aliphatics. Alkenes are similar but their molecular structure includes double bonds. Examples are ethylene and propylene. Alkynes contain triple bonds. Example is acetylene Aromatic hydrocarbons such as benzene have a ring molecular structure and burn with a smoky flame. When hydrocarbons burn they react with oxygen from the atmosphere to produce carbon dioxide and water although if the combustion is incomplete because there is insufficient oxygen, carbon monoxide will result as well. More complex organic compounds contain elements such as oxygen, nitrogen, sulphur, chlorine, bromine or fluorine and if these burn, the products of combustion will include other compounds as well. For example substances containing sulphur such as oil or coal will result in sulphur dioxide whilst those containing chlorine such as methyl chloride or polyvinyl chloride PVC will result in hydrogen chloride. In most industrial environments where gas detection is required, there is the risk of explosion or fire because of the presence of flammable gases or vapours, a mixture of compounds is likely to be encountered. In the petrochemical industry, the raw materials are a mixture of chemicals many of which are decomposing naturally or are being altered, by the processes. Saints Row 2 Full Rip Torrent here. For example crude oil is cracked to produce many simpler materials. Explosions. In order for gas to ignite there must be an ignition source typically a spark, or flame or hot surface. For ignition to take place there must be an explosive mixture. This means the concentration of gas or vapour in air must be at a level such that the fuel and oxygen can react chemically. The power of the explosion depends on the fuel and its concentration in the atmosphere. This is why you would use a Crowcon Combustible Gas Monitor to ensure safe levels are kept to protect staff, equipment and the surrounding environment. Not all concentrations of flammable gas or vapour in air will burn or explode. The LOWER EXPLOSIVE LIMITLEL The lowest concentration percentage of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source arc, flame, heat. The term is considered by many safety professionals to be the same as the lower flammable limit LFL. At a concentration in air lower than the LEL, gas mixtures are too lean to burn. Portable gas detectors in Australia have set 5LEL as its first alarm. The UPPER EXPLOSIVE LIMIT UEL Highest concentration percentage of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source arc, flame, heat. Concentrations higher than UFL or UEL are too rich to burn. Flammable liquids generally have a low FLASH POINT. This is the lowest temperature at which vapour is given off at sufficient rate to form an explosive mixture with air. Liquids with flash points below normal ambient temperatures automatically release vapour in sufficient volume to provide an explosive mixture, so leakage of such liquids is potentially as dangerous as a flammable gas leak. VAPOUR DENSITY is a measure of the density of a gas or vapour relative to air. Gases or vapours with a vapour density of less than one are lighter than air and they tend to rise from the point of escape and may therefore be readily dispersed or they may be trapped at a higher level. Gases or vapours with a vapour density of greater than one are heavier than air and tend to sink to lower levels and will spread around forming concentrations between the LEL and UEL.